Isaac Asimov: Life and Energy
Copper Extraction
Copper atoms tend to bond loosely with oxygen and sulfur forming certain bluish rocks. When these rocks are heated with carbon, the carbon removes the oxygen and sulfur atoms in the form of fire smoke leaving behind copper in its metallic state.
3500 B.C. copper smelting was a well established industry in Near East.
Tags:
Source:
Isaac Asimov: Life and Energy
Copper Smelting Origins
Copper smelting was likely discovered when campfire was accidentally built on some copper ore. The carbon from the burning wood extrated the sulfur and oxygen, leaving nuggets of cupper behind.
3500 B.C. copper smelting was well established industry in Near East.
Tags:
Source:
Isaac Asimov: Life and Energy
Bronze
Bronze is a copper tin alloy.
Tags:
Source:
Isaac Asimov: Life and Energy
Gun Powder
Gun powder was revolutionary because it could ignite without the presense of air. The combination of charcoal, sulfur and potassium nitrate, when heated, burned violently. This is because potassium nitrate contains loosely bound oxygen.
Tags:
Source:
Isaac Asimov: Life and Energy
First Cannon
Primitive cannon were supposedly first used at the battle of Crecy in 1346.
Tags:
Source:
Isaac Asimov: Life and Energy
Magnetism
The word magnetism is derived from Magnesia, a Greek city where stones with magnetic properties were first found.
Tags:
Source:
Isaac Asimov: Life and Energy
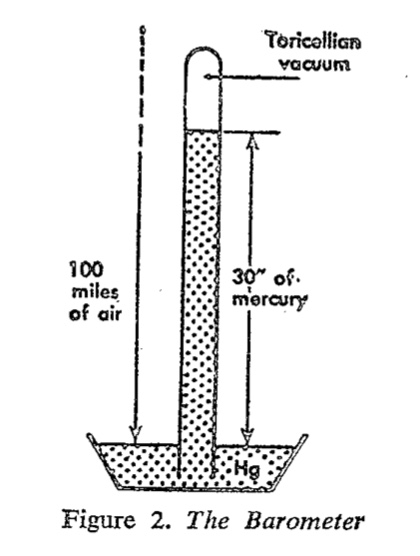
Barometer
Archimedes failed to explain why a column of water can be pumped only up to 10 meters of height. In the 17h century, Torcelli explained the phenomenon: Water in a tube that is closed at the top and sunk into a water reservoir at the bottom will rise only to such height that the weight of the water column will equal the weight of the atmospheric air column pushing on the reservoir. He tested the hypothesis using mercury, which should rise to even lower height because of its higher density. He was the first person to create vacuum and invented the barometer.
Tags:
Source:
Isaac Asimov: Life and Energy
Denis Papin
Papin was the first person to use the combination of steam and vacuum to do work. He filled a chamber with steam, then used cold water to cool the chamber, which condensed the steam in it and created a vacuum. The vacuum then "pulled" on a piston, doing work. It was the first demonstration of work done by not involving muscle power.
Tags:
Source:
Isaac Asimov: Life and Energy
Thomas Newcomen
Thomas Newcomen invented a water pump based on Papin's principle of filling a chamber with steam and then cooling the steam to condense it, thereby forming vacuum. The vacuum pulled a piston and lifted a column of water. This device was extremely inefficient, but was used in mines to pump out seeping water.
Tags:
Source:
Isaac Asimov: Life and Energy
James Watt & Steam Engine
James Watt improved Thomas Newcomen's steam pump be introducing a second chamber. One chamber was kept permanently cold (condenser), the other chamber permanently warm (cylinder). The steam was led into the cylinder chamber. That way, the cooled single chamber didn't have to be reheated after each cooling cycle. This significantly increased steam engine efficiency.
He also added a piston to both chambers so that the engine works in both directions.
Lastly, he connected the piston to a wheel, allowing the work done by the steam engine to be applied in wide variety of applications outside just water pumping. This started the Industrial Revolution.
Tags:
Source:
Isaac Asimov: Life and Energy
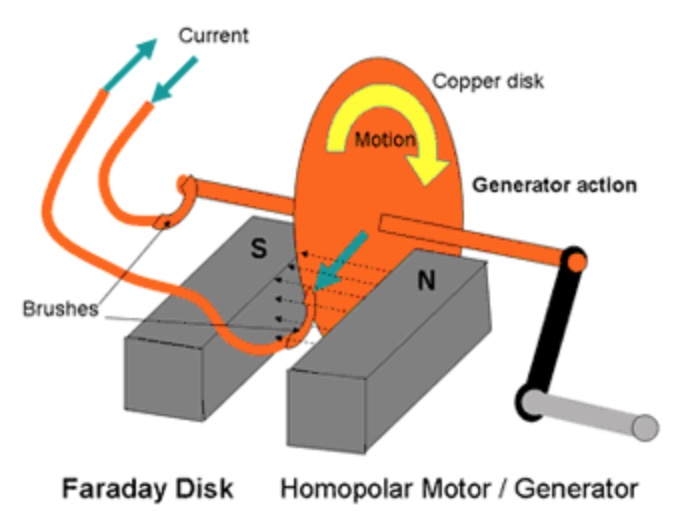
Michael Faraday & Electric Generator
In 1831, Faraday discovered that when a copper disk is rotated between the poles of a magnet, a continous electric current is generated. He created the first electric generator.
Tags:
Source:
Isaac Asimov: Life and Energy
Joseph Henry & Electric Motor
Joseph Henry invented the first iteration of the electric motor.
Tags:
Source:
Isaac Asimov: Life and Energy
Newton's First Law
Every body persists in its state of rest or in a uniform motion in a straight line unless it is compelled to change that state by forces impressed on it.
Tags:
Source:
Isaac Asimov: Life and Energy
Mass
Mass is the property of a body that describes to what extent the body will be accelerated under influence of force.
Tags:
Source:
Isaac Asimov: Life and Energy
Newton's Second Law
A force exerted on a body will produce an acceleration on the body directly propotional to the force and inversely proportional to the mass of the body.
Tags:
Source:
Isaac Asimov: Life and Energy
Newton's Third Law
For every action or force, there is an equal but opposite reaction or counterforce.
Tags:
Source:
Isaac Asimov: Life and Energy
Newton's Law of Universal Gravitation
All bodies in the universe attract all other bodies. The force between two bodies is directly proportional to the product of their masses and inversely proportional to the square of the distance between them.
Tags:
Source:
Isaac Asimov: Life and Energy
Dyne
A dyne is a quantity of force that when imparted to a body of mass 1 gram, it will accelerate the body by 1 cm/s2. Dynes are therefore measured in units of g*cm/s2. One Newton (kg*m/s2) is equal to 100,000 dynes.
Tags:
Source:
Isaac Asimov: Life and Energy
Erg
If we apply the force of one dyne over a distance of 1 cm, we do work of 1 erg (dyne-cm). One Joule (Newton-meter) is 10,000,000 ergs.
Tags:
Source:
Isaac Asimov: Life and Energy
Mechanical Energy Conservation
The sum of kinetic and potential energy of a closed system is constant. One form of energy can convert to another, but energy cannot be destroyed or created.
Tags:
Source:
Isaac Asimov: Life and Energy
Conservation of Momentum
The law of conservation of momentum was discovered in 1671 by John Wallis. He noticed that when objects collided, it wasn't their velocities that were preserved after the collision, it was the product of the objects' velocities and their masses that was preserved. Such property is called momentum.
Tags:
Source:
Isaac Asimov: Life and Energy
Temperature
Temperature is to heat what potential energy is to mass. Heat flows from regions of high temperature to those of low temperature.
Tags:
Source:
Isaac Asimov: Life and Energy
Air Thermometer
In 1592 Galileo invented an air thermometer, which measured temperature based on an expanding column of air in a tube in response to changing temperature. Unfortunately, the device was also sensitive to changes in air pressure, so it wasn't very accurate.
Tags:
Source:
Isaac Asimov: Life and Energy
Fahrenheit: Mercury Thermometer
In 1715, Gabriel Denis Fahrenheit deviced a new type of thermometer that used mercury in an enclosed tube to measure temperature. This is the thermometer common in household use in the 20th century.
Tags:
Source:
Isaac Asimov: Life and Energy
Joseph Black, Specific Heat
Joseph Black studied how much heat needs to be supplied to a substance to raise a unit mass of it by a unit of temperature. He called such property of the substance specific heat. The specific heat of water is arbitrarily set at 1.
Tags:
Source:
Isaac Asimov: Life and Energy
Specific Heat
A hot substance generally has a higher specific heat than a cool substance.
Tags:
Source:
Isaac Asimov: Life and Energy
Calorie
One calorie represents the amount of heat necessary to raise one gram of water from 14.5 to 15.5 degrees Celsia.
Tags:
Source:
Isaac Asimov: Life and Energy
British Thermal Unit
The British thermal unit (BTU) represents the amount of heat necessary to raise the temperature of 1 pound of water from 63 to 64 degrees Fahrenheit. 1 BTU equals 252 calories.
Tags:
Source:
Isaac Asimov: Life and Energy
Latent Heat
Joseph Black discovered a physical property called latent heat. Through measurement, he noticed that when he was heating a cube of ice, its temperature didn't rise as heat was added until it was fully melted. The amount of heat that had to be added to the ice cube before it was fully melted nearly equaled to the amount of heat that would bring the same quantity of ice-cold water to the boiling point. Today, this "hidden" (hence latent) heat is referred to as heat of fusion.
Tags:
Source:
Isaac Asimov: Life and Energy
Water/Mercury Expansion
Water doesn't just uniformly expand when heated. It actually contracts when transitioning between 0 and 4 degrees Celsia. Mercury uniformly expands as heated, which is why it has become popular for thermometer construction.
Tags:
Source:
Isaac Asimov: Life and Energy
Charles Boyle
Charles Boyle showed that the pressure and volume of a gas are inversely proportional. He did not consider the effects of temperature on either, though. This relationship is called Boyle's law.
Tags:
Source:
Isaac Asimov: Life and Energy
Charles' Law
Alexandre Charles showed that gases expand and contract uniformly in proportion to their temperature. Inreasing temperature expands the gas, decreasing temperature contracts the gas. This relationship is called Charles' law.
Tags:
Source:
Isaac Asimov: Life and Energy
Gas Expansion/Reduction
Alexandre Charles noticed that as gas temperature was increased or reduced by one degree Celsia, its volume expanded or contracted by 1/273 of its volume. This led to the eventual discovery of the absolute zero temperature concept.
Tags:
Source:
Isaac Asimov: Life and Energy
Lord Kelvin: Absolute Zero
Lord Kelvin introduced the Kelvin scale of temperature, substance containing no heat at all is at 0 degrees Kelvin, water freezes at 273 degrees Kelvin and boils at 373 degrees Kelvin.
Tags:
Source:
Isaac Asimov: Life and Energy
John Rankine Scale
Similar to Kelvin temperature scale, John Rankine devised an absolute zero temperature based scale, but using units of Fahrenheit instead of Celsius. On Rankin's scale, water freezes at 492 degrees and boils at 672 degrees. This so called Rank scale never got very popular.
Tags:
Source:
Isaac Asimov: Life and Energy
Thomas Young: Energy
The term energy was introduced by Thomas Young in 1807. He studied mostly nature of light.
Tags:
Source:
Isaac Asimov: Life and Energy
James Joule
James Joule studied how doing work on a liquid influences the liquid's temperature. He found out that no matter what form of energy source he applied to water (mechanical, electrical, chemical), the amount of work done increased the temperature of water by corresponding amount. Energy was conserved. Turns out that 41,800,000 ergs of work are equal to 1 calorie of heat. In Joule's honor, 10,000,000 ergs of work was expressed as a new unit of 1 Joule.
Tags:
Source:
Isaac Asimov: Life and Energy
Ludwich Hemholtz, Conservation of Energy
Ludwich Hemholtz is the first physicist to formally state the principle of conservation of energy, which he published in a paper in 1847.
Tags:
Source:
Isaac Asimov: Life and Energy
Antoine Lavoisier
Lavoisier conducted experiments carefully measuring the mass of substances involved in chemical processes such as combustion an rusting. He found out that the total mass of all involved agents remained the same prior and after the reaction. In other words, mass is conserved. Just like energy, mass cannot be created or destroyed.
Tags:
Source:
Isaac Asimov: Life and Energy
Einstein: Mass to Energy Conversion
In 1905 Albert Einstein showed that mass had to be considered a form of energy through his famous equation E=mc2.
Tags:
Source:
Isaac Asimov: Life and Energy
Perpetuum mobile
As early as in 1775, the Paris Academy of Sciences refused to patent any device that claimed that it could generate perpetual motion without any apparent energy source. The laws of thermodynamics were about to be established and firmly understood by the scientific society.
Tags:
Source:
Isaac Asimov: Life and Energy
Watt's Steam Engine
Even the best of Watt's steam engines didn't reach efficiency higher than 5%.
Tags:
Source:
Isaac Asimov: Life and Energy
Carnot: Efficiency
Carnot showed that the maximum theoretical efficiency obtainable by a machine can be found from the temperatures of the hot and cool reservoirs involved in heat exchange. The expression is: Efficiency = (Th - Tc) / Th.
Tags:
Source:
Isaac Asimov: Life and Energy
Second Law of Thermodynamics
The only way to convert heat into work is if there is a difference in temperatures somewhere in the systems.
Tags:
Source:
Isaac Asimov: Life and Energy
Julius Clausius
Clausius, in 1850, explicitly stated that heat can only flow from a region of higher temperature to that of lower temperature.
Tags:
Source:
Isaac Asimov: Life and Energy
Entropy
In any system converting one form of energy to another, some energy is lost due to inefficiencies such as friction or some other form of resistance to energy flow. Clasius named this property entropy. In any spontaneus process, entropy either remains the same or it increases.
Tags:
Source:
Isaac Asimov: Life and Energy
Entropy
Entropy always increases in any process when the full system involved is considered. Even if locally, entropy decreases due to work being done on some part of the system, that entropy decrease is more than outweighted by an increase in entropy elsewhere in the system.
Tags:
Source:
Isaac Asimov: Life and Energy
Heat-Death of Universe
Since over time entropy increases in the universe, different regions of universe reach the same temperature. At that point, there is no potential for spontaneous heat flow. The universe cannot do work anymore and reaches a state called heat-death. This was a popular theory of cosmology in the 19th century.
Tags:
Source:
Isaac Asimov: Life and Energy
Heat as Motion
Galileo and Newton already speculated that heat might represent a motion of small subdivisions of matter.
Tags:
Source:
Isaac Asimov: Life and Energy
Imponderable Heat Fluid
In the 18th century, heat was envisioned as a flow of an invisible "imponderable" fluid called the caloric. In 1770, Lavoisier overthrew this theory.
Tags:
Source:
Isaac Asimov: Life and Energy
Phlogiston
Phlogiston was a speculated substance that, in the 18th century, was believed to escape matter when it rusted or burned.
Tags:
Source:
Isaac Asimov: Life and Energy
Count Rumford
Count Rumford challenged the caloric fluid theory when he observed drill bits at work. He noticed that the bits seem to have an endless supply of the caloric fluid and also the duller the bits get, the more heated they get, therefore, more caloric seams to be seaping out of the bit. He was convinced that the heat was the result of the work done by the bit, not coming from the imponderable caloric.
Tags:
Source:
Isaac Asimov: Life and Energy
Humphry Davy
In 1799, Humphry Davy ran an experiment where he used a mechanical device to rub two pieces of ice against each other in sub freezing temperature environment. According to the old theory, ice should contain no caloric fluid at that temperature, so no heat should be released. However, he observed that the two ice pieces started to melt when rubbed against each other. He concluded that the rubbing motion generates heat.
Tags:
Source:
Isaac Asimov: Life and Energy
John Dalton: Atom Theory
In 1803, John Dalton proposed a modern version of the theory of atoms, which was inititally proposed by the ancient Greeks. The modern scientific society was skeptical of existence of particles this small. Dalton considered atoms indivisible and indestructible.
Tags:
Source:
Isaac Asimov: Life and Energy
Joule-Thompson Effect
When a gas expands into vacuum, the atoms in it have to overcome some forces holding them together. As a result, gas expanding into vacuum actually does some work (to overcome the attractive forces), which causes its temperature to slightly decrease. Thompson was Lord Kelvin's original name. This attractive force is ignored in the ideal gas law.
Tags:
Source:
Isaac Asimov: Life and Energy
Ideal Gas
Ideal gas is one where, for theoretical purposes, constituent atoms are considered to have no volume (point particles) and no attractive forces among them.
Tags:
Source:
Isaac Asimov: Life and Energy
Ideal Gas Law
Boyle's and Charle's law apply only for ideal gases. When combined, the resulting law relates temperature, volume, and presure of gas. This is called the equation of state.
Tags:
Source:
Isaac Asimov: Life and Energy
Johannes var der Waals
Johaness van der Waals introduced a small change to the equation of state (ideal gas law) that accounted for some of the neglected gas properties, such as non-zero volume of atoms and interatomic attractive forces.
Tags:
Source:
Isaac Asimov: Life and Energy
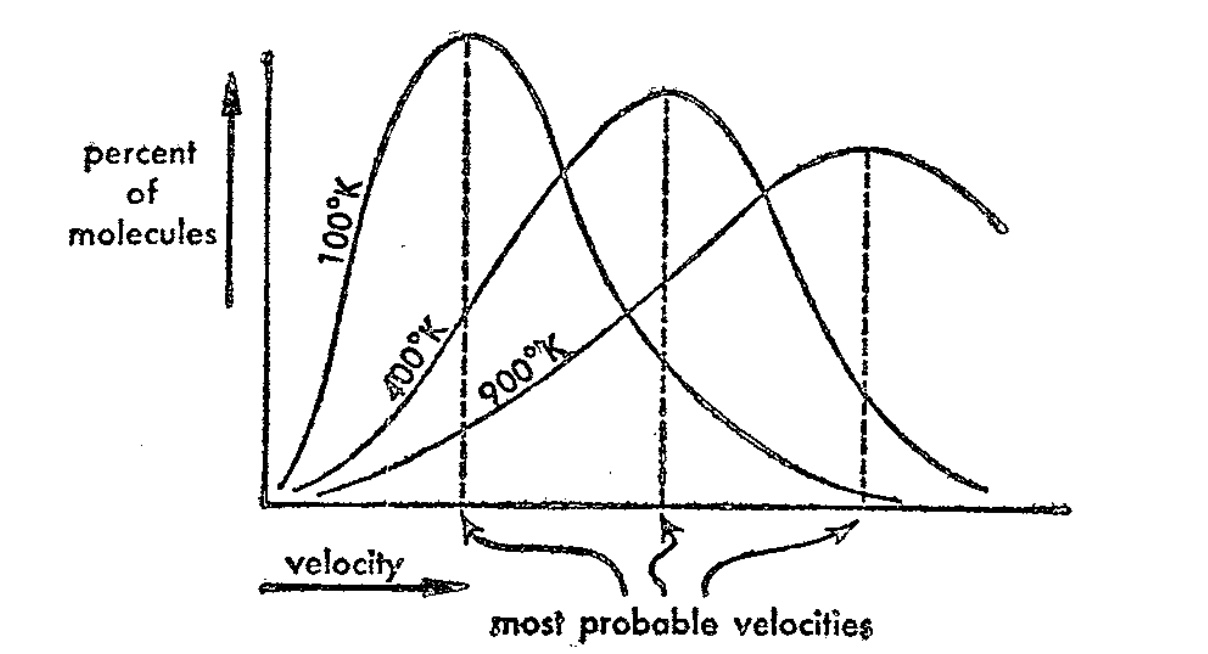
Maxwell-Boltzmann Equation
Maxwell and Boltzmann derived an equation that calculates what fraction of molecules in a gas move at a particular velocity at a given temperature.
Tags:
Source:
Isaac Asimov: Life and Energy
Kinetic Energy of Gases
At any given temperature the average kinetic energy of molecules of all gases is equal. Heavier gas molecules move slower than ligher gas molecules, but their energies are equal. Temperature, then, is the average kinetic energy of the molecules making up a gas.
Tags:
Source:
Isaac Asimov: Life and Energy
Maxwell's Demon
Maxwell came up with the concept of Maxwell's Demon, a theoretical being that selectively let gas molecules move through a small opening in a wall only in one direction (like a diode for gas flow), but not in the other direction. That way, the random motion of molecules in a gas could be organized such that all molecules end up in a hot compartment instead of naturally expanding through all space available to them and cooling down in the process. In other words, energy could be extracted from the system. This is just theoretical and is practically not doable.
Tags:
Source:
Isaac Asimov: Life and Energy
Brownian Movement
Brownian movement is the random motion of particles suspended in a substance. The random jittery motion of the molecules making up the substance jolt the particles around. This phenomenon was observed by the botanist Robert Brown when he observed such jittery motion on pollen particles suspended in water. This observation played an important role in the establishment of the modern molecule theory.
Tags:
Source:
Isaac Asimov: Life and Energy
Einstein: Brownian Motion
In 1905, Albert Einstein worked out a thorough mathematical treatment of the Brownian motion of molecules. In the 1920's and 1930's, experiments measured velocities of molecules in gases and showed that their velocities were in agreement with the kinetic theory of gases.
Tags:
Source:
Isaac Asimov: Life and Energy
Entropy and Disorder
Entropy and disorder are analogous phenomena.
Tags:
Source:
Isaac Asimov: Life and Energy
Coal Burning & Phlogiston
In the 18th centry, the heat liberated during burning of coal was explained as the escape of the imponderable fluid called phlogiston.
Tags:
Source:
Isaac Asimov: Life and Energy
Chemical Energy of Water vs Ice
Liquid water at 0 degrees Celsia contains more chemical energy than ice at 0 degrees Celsia. This is because while ice is melting, the added heat is being converted into chemical energy instead of kinetic energy. The chemical energy represents the energy needed to break the bonds holding the solid ice water molecules together. As heat is added, the energy goes into breaking those bonds instead of to kinetic energy. That's why the melt water of ice is at the same temperature as the ice until all of the ice melts. Melt water at 0 degrees contains the same kinetic energy as ice at 0 degrees plus the chemical energy.
Tags:
Source:
Isaac Asimov: Life and Energy
Vaporization
At any temperature, liquid water (or any liquid) is always vaporizing to some extent. If the liquid is sealed, the vapor creates a pressure resulting in some of the vapor condensing back to water. At some vapor pressure, the vapor condensation and evaporation reach an equlibrium point. The higher the temperature, the greater the pressure.
Tags:
Source:
Isaac Asimov: Life and Energy
Vapor Pressure & Boiling Point
At standard atmospheric pressure and temperature, the vaper pressure of a pot of water is far lower than the pressure of the atmosphere "pushing" (condensing) the vapor back to liquid form. When temperature of water reaches 100 degrees Celsia, the vapor pressure is higher than the atmospheric pressure and vapor starts rapidly escaping from the liquid creating the boiling effect. At higher altitudes, the atmospheric pressure is lower, therefore the vapor pressure exceeds the atmospheric pressure at lower temperatures.
Tags:
Source:
Isaac Asimov: Life and Energy
Energy in Water vs Steam
One gram of steam at 100 degrees Celsia has 540 calories more chemical energy than one gram of water at the same temperature. This is due to the latent heat of vaporization. One gram of water cooling from 100 degrees Celsia to 0 degrees releases 100 calories. Therefore, most of the work obtained from a steam engine is obtained from steam condensing to water, not from water cooling.
Tags:
Source:
Isaac Asimov: Life and Energy
Vaporization & Sweating
Water vapor at any temperature contains more energy than liquid water at the same temperature. It is for this reason that perspiration acts to cool the body. The chemical energy necessary to form the evaporating sweat is extracted from the body.
Tags:
Source:
Isaac Asimov: Life and Energy
Water Breakdown Temperature
At about 3000 degrees Celsia, about 1/4 of water molecules have broken up into hydrogen and oxygen.
Tags:
Source:
Isaac Asimov: Life and Energy
Atomic Weights
It was from the proportion by weight in which various elements combined that it was first possible to work out the atomic weights of the various elements.
Tags:
Source:
Isaac Asimov: Life and Energy
Avogadro's Number
Represents the number of hydrogen molecules (two atoms each) in two grams of mass of pure hydrogen.
Tags:
Source:
Isaac Asimov: Life and Energy
Gram Molecular Weight: Mole
Gram molecular weight is the weight equal to the molecular weight in grams. It is customarily abbreviated as "mole". A mole of hydrogen weighs 2 grams, a mole of oxygen weighs 16 grams. A mole of water, therefore, weighs 18 grams.
Tags:
Source:
Isaac Asimov: Life and Energy
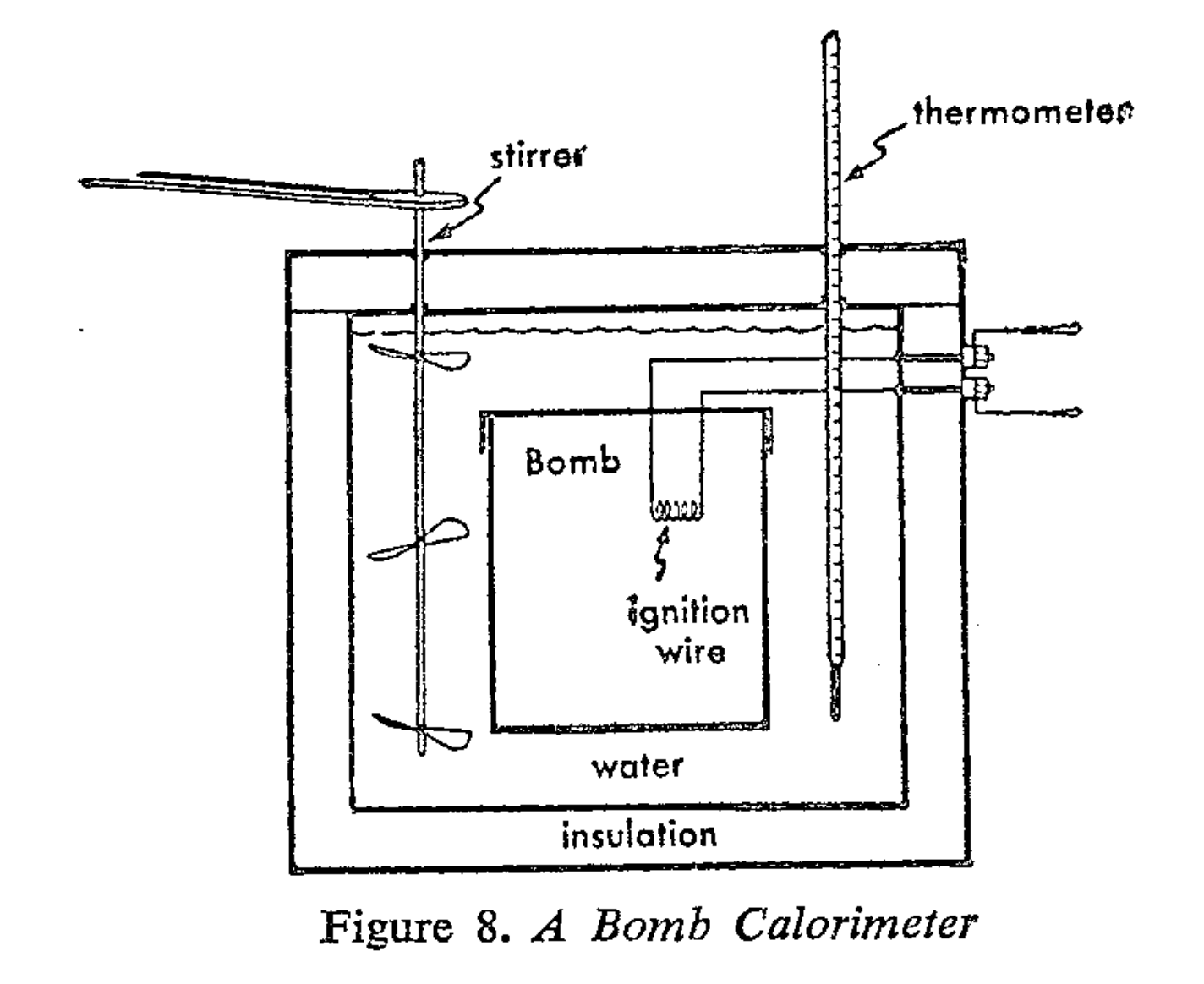
Bomb Calorimeter
The bomb calorimeter was a device to measure how much heat was released or consumed during chemical reactions.
Tags:
Source:
Isaac Asimov: Life and Energy
Energy Under Constant Volume
The change in energy of a system under constant volume (changing pressure) is symbolized using ΔE. In modern literature, it's often denoted ΔU.
Tags:
Source:
Isaac Asimov: Life and Energy
Volume of Mole of Gas
One mole of any gas at standard temperature and pressure occupies 22.4 liters of volume.
Tags:
Source:
Isaac Asimov: Life and Energy
Enthalpy
Energy change due to a chemical reaction is greater under contant pressure than under constant volume. This energy change is denoted ΔH, is called enthalpy, and the quantity is always greater than ΔE, energy change under constant volume. This is because an expanding gas does work against the atmosphere and can later reclaim the work. Hence, the atmosphere acts as an energy reservoir.
Tags:
Source:
Isaac Asimov: Life and Energy
Heat of Reaction
The amounts of reactants in a reaction for the sake of the measurement of the heat of the reaction is best measured in moles rather than grams. That way, equal numbers of molecules are involved from each ractant even if their masses are different. Therefore, ΔH stands for molar heat of reaction at constant pressure. Enthalpy (ΔH) is written on a side of the reaction like this. If energy is released, the number is negative. If energy is absorbed, the number is positive.
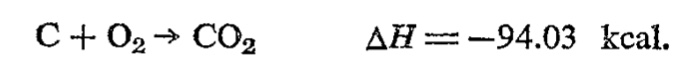
Tags:
Source:
Isaac Asimov: Life and Energy
Hess' Law
If a reaction is carried out in stages, the sum of heats of reaction of each stage is equal to the heat of reaction if the reaction was carried out completely in a single step. This is important because heats of reactions of certain chemical reactions cannot be directly observed. They can be indirectly calculated using Hess' law from multi-step reactions that can be observed, though.
Tags:
Source:
